Abstract
Introduction:
Itolizumab is a first-in-class monoclonal antibody against the co-stimulatory receptor CD6 that blocks its interaction with activated leukocyte cell adhesion molecule (ALCAM), thereby inhibiting T effector (T eff) cell activity and trafficking to target organs. It is being evaluated as a treatment for immuno-inflammatory diseases where T eff cells play a central role including acute graft-versus-host disease (aGVHD). Previous studies reported that ex vivo depletion of donor CD6+ cells in allogeneic hematopoietic cell transplantations lowers the incidence of aGVHD, justifying therapeutically targeting CD6 in aGVHD. Here we present interim clinical and pharmacokinetic/dynamic (PK/PD) results from the EQUATE study (NCT03763318), an ongoing US-based Phase 1b/2 study of itolizumab (a non-depleting anti-CD6 mAb) to treat subjects with newly diagnosed aGVHD, highlighting the relationship of early response to itolizumab concentrations.
Methods:
To date, 22 adult subjects with Grade III-IV aGVHD who initiated steroid treatment within 7 days prior to the first dose of itolizumab have enrolled in EQUATE at 0.4 mg/kg (n=4), 0.8 mg/kg (n=9), or 1.6 mg/kg (n=9), administered IV every 2 weeks x 5 doses. The median follow-up is 146 days (range: 14-355 days). Primary endpoints included itolizumab safety, tolerability, and optimal dose levels, and secondary endpoints included PK/ PD effects (change in CD6 surface expression on CD4+ cells) and clinical activity.
Results:
Patients: At baseline, study subjects had a mean (SD) age of 54 (14) and 68% were male; all had Grade III or IV aGVHD and 91% had lower GI involvement. All subjects received at least one dose and 15 received at least 2 doses of itolizumab.
Safety: All subjects experienced at least 1 AE. Serious AEs occurred in 14 subjects (64%), with 9 (41%) reporting infection-related SAEs. There were 8 deaths. Six subjects (27.3%) had SAEs leading to death (3 at 0.8 mg/kg and 3 at 1.6 mg/kg): sepsis (n=1), Staphylococcal sepsis (n=1), Klebsiella sepsis (n=1), intestinal infarction (n=1), cardiac arrest (n=1), and GVHD (n=1). Another 2 deaths occurred >100 days post- last dose due to progressive aGVHD (n=1) and primary disease relapse (n=1).
Efficacy and Survival: Across all doses, the complete response (CR) rate was 55% at both Day 15 and Day 29, and the overall response rate (ORR) was 73% at Day 15 and 68% at Day 29. At Day 169 (n=20), non-relapse mortality (NRM) was 35%, overall survival was 65%. Of note, the 10 subjects who achieved an early CR at Day 15 had a lower rate of NRM at Day 169 (20%) compared to the 10 subjects who had a very good partial response (VGPR), partial response (PR), no response (NR), or disease progression (50%).
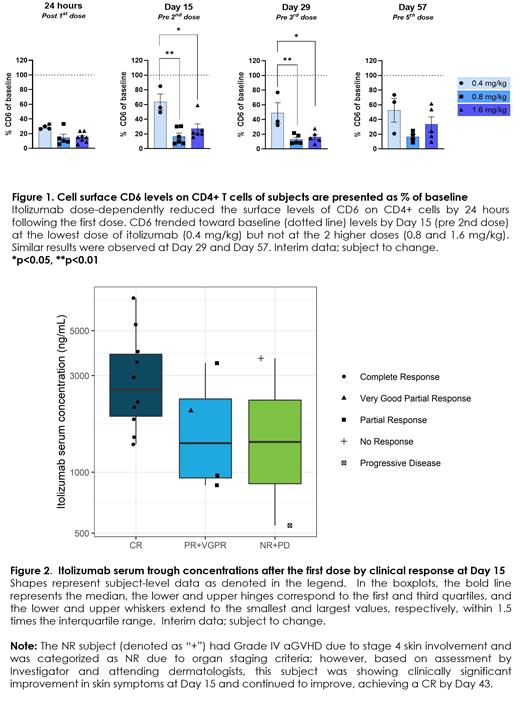
PK/PD: Itolizumab substantially decreased the levels of cell surface CD6 on circulating T cells after the first dose in a dose-dependent manner and maintained that decrease throughout the treatment period (Figure 1). Notable findings by immunophenotyping included: (1) an increase in the ratio of T regulatory to T eff cells at 0.8 and 1.6 mg/kg dose-level by Day 15, and (2) decreases of PD-1 expression on T cells and of ALCAM on CD14+ monocytes at all dose-levels by Day 8. This indicates itolizumab can reduce activation of T eff cells and monocytes. The relationship between drug concentrations and efficacy was evaluated at Day 15 when there was greater variability in response relative to later timepoints. Higher itolizumab trough concentrations on Day 15, achieved at higher dose-levels, correlated with a higher rate of CR at Day 15 (Figure 2).
Conclusions:
In summary, the observed safety, efficacy, PK and PD to date from this ongoing study indicate a favorable benefit-risk profile in subjects with Grade III-IV aGVHD. The relationship between itolizumab concentrations after the first dose and clinical response suggest that higher itolizumab exposures early (by Day 15) are impactful for longer term clinical responses. These data support the design and initiation of a pivotal phase 3, placebo-controlled clinical trial to assess itolizumab in combination with corticosteroids as first-line treatment of aGVHD.
Koreth: Equillium: Research Funding; Regeneron: Research Funding; Clinigen Labs: Research Funding; BMS: Research Funding; Miltenyi Biotec: Research Funding; Gentibio Inc.: Consultancy; EMD Serono/Merck: Consultancy; Amgen: Consultancy; Moderna: Consultancy; Cugene: Other: Scientific Advisory Board; Mallinckrodt: Other: Scientific Advisory Board; Biolojic Design: Other: Scientific Advisory Board. Ritz: Amgen: Research Funding; Equillium: Research Funding; Kite/Gilead: Research Funding; Avrobio: Membership on an entity's Board of Directors or advisory committees; Akron: Consultancy; Biotech: Consultancy; Blackstone Life Sciences Advisor: Consultancy; Clade Therapeutics, Garuda Therapeutics: Consultancy; Immunitas Therapeutic: Consultancy; LifeVault Bio: Consultancy; Novartis: Consultancy; Rheos Medicines: Consultancy; Talaris Therapeutics: Consultancy; TScan Therapeutics: Consultancy. Chinn: Equillium: Current Employment, Current equity holder in publicly-traded company; Principia Biopharma: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months; Genentech/Roche: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months, Patents & Royalties: Methods of treating immune diseases using an inhibitor of Bruton's tyrosine kinase (provisional patent application. Ng: Equillium: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Acevedo: Equillium: Current Employment, Current equity holder in publicly-traded company; Arena: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Chu: Equillium: Current Employment. Fung: Equillium: Current Employment; Arena: Ended employment in the past 24 months, Patents & Royalties. Rothman: Equillium: Current Employment, Current equity holder in publicly-traded company. Connelly: Equillium: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months, Membership on an entity's Board of Directors or advisory committees. Thomas: Equillium: Current Employment, Current equity holder in publicly-traded company; Chinook: Current equity holder in publicly-traded company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Principia: Current equity holder in publicly-traded company, Ended employment in the past 24 months. Cutler: Deciphera: Consultancy; Cimeio: Consultancy; Editas: Consultancy; Kadmon: Consultancy; Pfizer: Consultancy; Mallinckrodt: Consultancy; CareDx: Consultancy; Incyte: Consultancy; Omeros: Consultancy; Syndax: Consultancy; Mesoblast: Consultancy; Jazz: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal